Chemical Processes
What is a redox reaction?
Simply, redox can be characterised by the presence of oxygen (oxic) or absence (anoxic) of oxygen. However, it is more accurately described as chemical reactions which involve the transfer of electrons. The chemical species which loses the electron (increase in oxidation state) is oxidised, while the chemical species that gains the electron (decrease in oxidation state) is reduced.
It may sound complex, but redox reactions are part of everyday life. For example, the stagnation of water, rusting of metal, electrical energy derived from batteries, and cellular respiration are all redox reactions.
In the natural environment redox reactions are driven by microbes (catalysed). In soil and aquifer materials there are trillions of bacteria, many of them specialising in redox reactions from which they derive energy. This myriad of bacteria drives carbon, nitrogen, and phosphorus cycling in the environment.
Watch the video below for an overview of redox processes.
How do redox reactions occur in the environment?
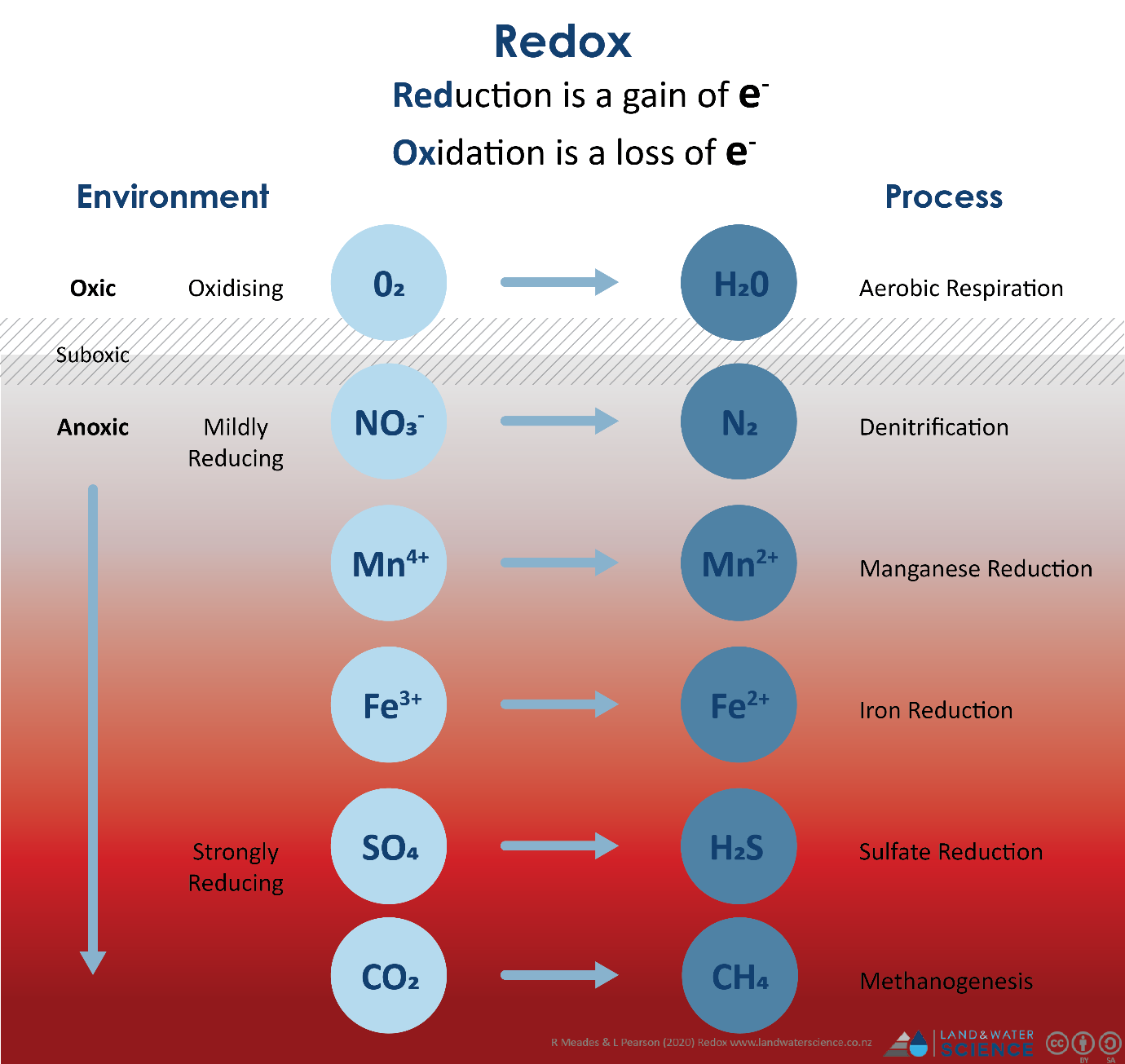
The process of stagnation of water in a pond or puddle is a classic example of redox in the environment. The same process occurs in soils, streams, and aquifers at a larger scale. As with any reaction process, how much stagnation occurs will vary depending on the availability of organic carbon, the main food source for microbes (the electron donor), and subsequently the availability of oxygen, nitrogen, manganese, iron, sulphate, and carbon dioxide (the electron acceptor).
Microbes are highly efficient (lazy), they prefer to expend as little energy as possible. When they consume their preferred food source, organic matter, they must break the chemical bonds between carbon atoms. Breaking bonds requires a lot of energy. To save energy, microbes use oxygen, which has a high affinity for electrons to help break the electron bonds. As the electrons are moved from the organic matter to oxygen, oxygen is consumed and converted to water (H₂O). If oxygen is always available for this process, the water will remain oxic and never stagnate (become reducing).
If the supply of oxygen runs out, microbes will quickly switch to using nitrate, which has the next highest affinity for electrons. The nitrate becomes reduced by gaining an electron and gets removed from the water as nitrogen gas (N₂) through a process called denitrification.
Once nitrate has been consumed, microbes then move onto using manganese (Mn), then iron (Fe), then sulphate (SO₄) as the electron acceptor. At each step in electron acceptor, it becomes progressively harder for the microbes to get energy and the water becomes more reducing. When microbes use sulphate as an electron acceptor, it gets converted to hydrogen sulphide, which is characterised by a rotten egg smell typical of strongly reducing stagnant water. Eventually, microbes may end up having to use carbon dioxide dissolved in water as an electron acceptor. In this case, the carbon dioxide is converted to methane (CH₄, a strong greenhouse gas). Methane is most commonly produced in wetlands where there is an abundance of organic carbon. If fresh oxygen or nitrate is introduced to the soil or water, microbes will quickly switch back to using these more energy efficient chemicals for accepting electrons.
Why is redox important for water quality?
Redox is important to a range of environmental concerns, including low dissolved oxygen in surface waters, nitrate leaching, denitrification, and phosphorus solubility and mobility. Where soils and aquifers have high oxygen, bacteria don’t need nor bother to use nitrate as an electron acceptor, and as a result it can build up in the environment. When oxygen is restricted, nitrate is commonly removed by bacteria (denitrified).
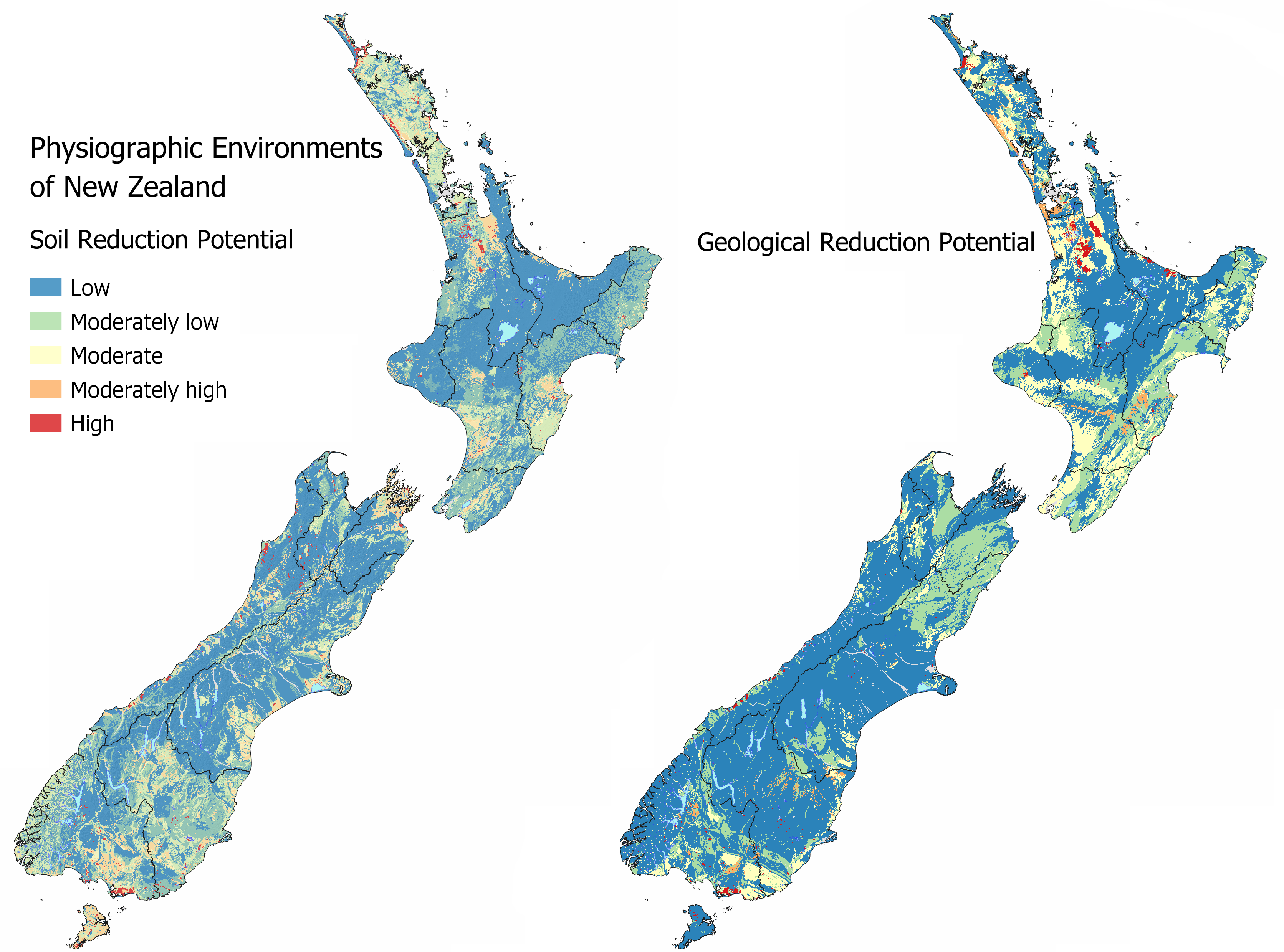
By mapping the likely redox potential of soil and geology we can predict where shallow groundwater is likely to contain elevated manganese, iron, and arsenic (in areas with arsenic bearing minerals), which limits the use of groundwater as a drinking water source.
Soil zone redox processes also determine the magnitude of soil zone greenhouse gas emissions (GHG), such as nitrous oxide and methane. When nitrate is used as an electron acceptor in the soil it may convert the nitrate to nitrous oxide, a potent greenhouse gas. The ‘A’ horizon of most soils is where the highest amount of nitrous oxide greenhouse gasses are produced. However, this only occurs when soils get saturated with water, which shuts off the supply of oxygen to soil microbes. As the soil drains and oxygen from the atmosphere is once again available, microbes shift to using oxygen as an electron acceptor and the production of nitrous oxide ceases.
How do you predict the redox potential of soil and geology?
Reduction potential can be used to predict the likelihood of redox reactions occurring in the environment. It is predicted by measuring the concentrations of oxygen, nitrate, manganese, iron, and sulphate in water samples and comparing the soil type and geological material at that location. As the electron acceptors are always used in that order, we have an idea of how reducing a soil or geological material may be.
In soils, how saturated the soil is and the abundance of organic carbon are the main predictors for redox potential. Saturation can be predicted upon soil properties such as drainage class, texture, permeability, and depth to slowly permeable layers. Carbon content can be measured in a laboratory and is mapped in our national soil databases. New Zealand soils don’t typically show a limitation in carbon content, therefore the degree of saturation (how long a soil is cut off from atmospheric oxygen) drives the redox potential. Soils with a low reduction potential (less reactive) are typically well drained (always oxygenated) while soils with a high reduction potential (highly reactive) are saturated all year round.
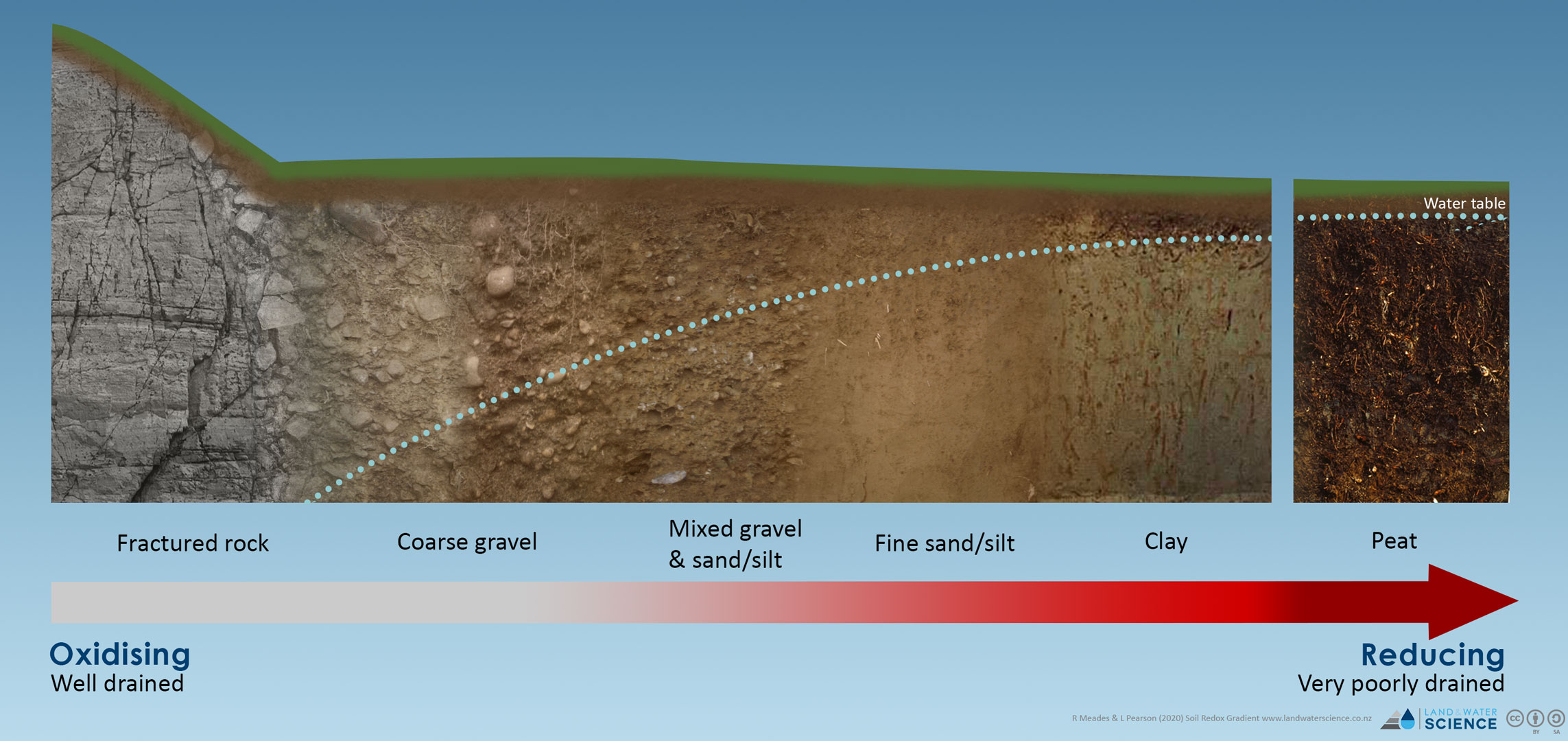
In aquifers, the carbon content of the geological material is a useful predictor of redox potential. Geological materials with low carbon content, such as sands and gravels have a low reduction potential, while sedimentary geologies (mudstones, marine derived sediment, lignite, and peat) have a higher carbon content and ultimately a higher reduction potential.
| Geological Unit | Geological Composition | Reduction Potential Potential |
|---|---|---|
| Active peat wetlands | Peat | High |
| Estuarine sediments | Estuarine sediments | High |
| Low rank coal measures | Lignite, alluvial gravels over lignite | Moderately high |
| Mixed marine-terrestrial terraces | Marine or ocean sediment | Moderate |
| Glauconitic limestone | Limestone with variable glauconite or greensand | Moderate |
| Carbonaceous sediments | Minor carbonaceous sediments (coal or carbon) | Moderate |
| Pyritic sediments | Sediments with variable pyrite | Moderate |
| Mudstones | Mudstone sediments (non-siliceous or argillic, poorly indurated) | Moderate |
| Minor muds | Muds (not mudstone) | Moderately low |
| River floodplains – minor peat | Alluvial gravels, sand, silt, and clay with minor peat | Moderately low |
| Modern river floodplains | Alluvial gravel, sand, silt and clay | Low |
| Terraces/outwash plains/Glacial deposits | Weathered gravel, sand, silt and clay | Low |
| High terrace and basin gravels | Highly weathered gravel, sand, silt and clay | Low |
| Volcanic deposits | Ignimbrite and other volcanic deposits | Low |
The soil and geological reduction potential are mapped independently of each other and can be viewed in MAPS – CHEMICAL PROCESSES. As redox processes occur in both the soil zone and underlying aquifer, it is important to know the hydrology also to establish how connected the landscape is for the reduction potential to be realised. For example, a poorly drained soil will have a moderately high to high reduction potential, however if that soil is artificially drained a lower reduction potential is predicted relative to the same soil unmodified by drainage as the contact and time water spends in the soil under saturation is significantly lower.
What is denitrification?
Denitrification is a series of redox reactions driven by microorganisms that deal specifically with the transformation of nitrogen from nitrate to dinitrogen gas. A large range of microbes in the environment can denitrify, however it only occurs in response to the depletion of oxygen (O₂) in their immediate environment. Only when oxygen is limited, will denitrifying microbes switch from aerobic respiration to anaerobic respiration, using nitrate (NO₃⁻) as the electron acceptor. Nitrate concentration also needs to be sufficient for denitrification to occur.
In aerated soils, microbes breakdown organic matter in the presence of oxygen to produce carbon dioxide (CO₂), water, and energy. Nitrate lost through the soil in these areas is more likely to accumulate in the groundwater as there is a low potential for nitrogen reduction from denitrification.
In very wet or waterlogged soils, oxygen is rapidly depleted, and the microbes use nitrate instead of oxygen for respiration. When more than 60–70 % of the pore space in soils are filled with water, oxygen is rapidly depleted by respiring bacteria. This condition lasts longer in poorly drained soils even when water is not necessarily visible on the land surface. Even after draining, clay soils can still have micro-sites that remain anaerobic compared to fully aerobic, lighter-textured sandy or loamy soils.
Denitrification can occur in any soil where internal drainage is restricted; for example, a soil with a high clay content that drains slowly, or sandy soil over an impervious layer, such as a pan, that impedes drainage. We typically see little nitrate discharged from bedrock sourced streams, as saturation leading to denitrification occurs at the contact between soil and the lower permeability bedrock. The rate of denitrification can be influenced by factors such as the amount of rain, duration of rain, how wet the soil profile is, and the capacity for the soil to drain internally.
The denitrification process involves the transformation from nitrate (NO₃⁺) to nitrite (NO₂⁻), to nitric oxide (NO), to nitrous oxide (N₂O), and finally resulting in the production of dinitrogen (N₂) completing the nitrogen cycle. Denitrification produces several gases: nitric oxide (NO), and nitrous oxide (N₂O) which are harmful greenhouse gases, then dinitrogen (N₂) the inert gas (non-reactive) that makes up 78 % of our atmosphere. When denitrification can proceed to completion dinitrogen is the main form of N gas that is lost, but the proportion of the different gases produced depends on soil pH and water content. Incomplete or partial denitrification occurs under suboptimal conditions when oxygen becomes depleted but is not fully anoxic. Once nitrogen is in a gas form, it is no longer available to plants in the soil.
Denitrification occurs at all temperatures but is slower in colder conditions and faster as it gets warmer. The rate increases exponentially between 15 °C and 30 °C, and peaks between 23 °C and 27 °C. Soil pH affects both how fast denitrification can occur (rate) and the products of denitrification. It is slow in acidic soil (low pH), and rapid at alkaline pH (high pH). In acidic soils, more nitrogen is lost as nitrous oxide while in alkaline soils, most nitrogen is lost as dinitrogen.
For more information on the nitrogen cycle see SCIENCE – WATER QUALITY CONTAMINANTS.
How do redox reactions mobilise phosphorus?
Phosphorus is found in soils in both an organic form and a mineral form and its solubility in soil is generally low. The types of phosphorus compounds that exist in the soil are mostly controlled by soil pH and by the type and amount of minerals in the soil. Mineral compounds containing phosphorus usually contain aluminium, iron, and manganese in acidic soils (low pH), and calcium in alkaline soils (high pH). It is the phosphorus adsorbed to mineral compounds containing iron or manganese that is likely to be mobilised under reducing soil or aquifer conditions. As iron is the most abundant element on the earth, it is highly likely to be controlling phosphorus mobility where redox conditions are favourable.
For this reason, phosphorus concentrations are often elevated in drainage waters from bedrock environments, reducing soil environments, and wetlands. How much phosphorus is mobilised is directly proportional to how reducing the environment is and the amount of organic matter (phosphorus source).
Phosphorus can also be mobilised by chemical weathering of phosphorus rich rock types in areas that are naturally elevated. This process is independent from the redox reactions described above.
For more information on the phosphorus cycle see SCIENCE – WATER QUALITY CONTAMINANTS.