Phosphorus
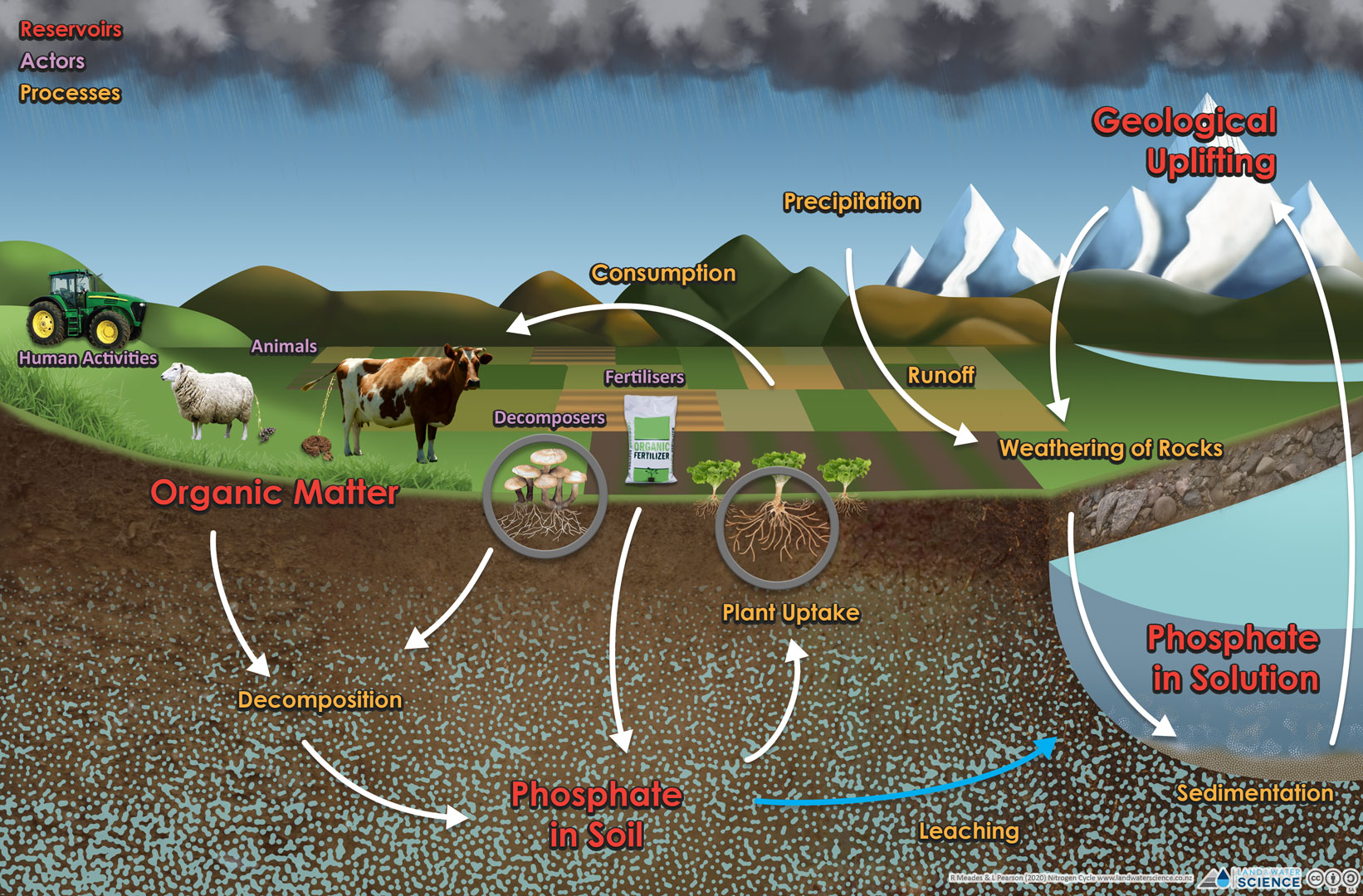
Phosphorus (chemical symbol P) is predominantly found as phosphate-based compounds (solid form) and is cycled through the lithosphere (the rigid outer surface of the earth), hydrosphere (all the water on the earth’s surface), and biosphere (the regions of the surface and atmosphere of the earth occupied by living organisms).
How is phosphorus cycled on my farm?
In the environment, the weathering of rocks and minerals releases phosphorus in a soluble form where it is taken up by plants, and subsequently transformed into organic compounds. On a farm, P this is typically added through the use of phosphate fertilisers. Unlike with nitrogen, the atmosphere does not play a significant role in the cycling of phosphorus.
In soil, phosphate is absorbed by iron oxides, aluminium hydroxides, clay surfaces, and organic matter particles, and becomes incorporated in the soil particle. When soil is lost by runoff, it takes the phosphorus with it. Organic phosphate is phosphorus that has been incorporated into plant or animal tissue (e.g., seeds, leaves).
In natural waters, phosphorus typically occurs in both inorganic and organic forms.
Why is too much phosphorus an issue?
Humans have had a significant impact on the phosphorus cycle due to a variety of human activities, specifically the use of fertiliser, the distribution of food products, and excessive nutrient concentrations in waterways (artificial eutrophication). P fertilisers increase the phosphorus levels in the soil and are particularly detrimental when lost into local aquatic ecosystems. When phosphorus is added to water at a rate typically achieved by natural processes, it is referred to as natural eutrophication. A natural supply of phosphorus over time provides nutrients to the water and serves to increase the productivity of the ecosystem. However, when levels of phosphorus are too high, the overabundance of plant nutrients enables the excessive growth of algae. A rapid increase in the population of algae in an aquatic ecosystem forms an algal bloom. Blooms can reduce the amount of light and oxygen available to other aquatic life. Some types of algae may be toxic if ingested or can be an irritant to skin and eyes.

Image credit: www.lawa.org.nz
How is phosphorus measured in water?
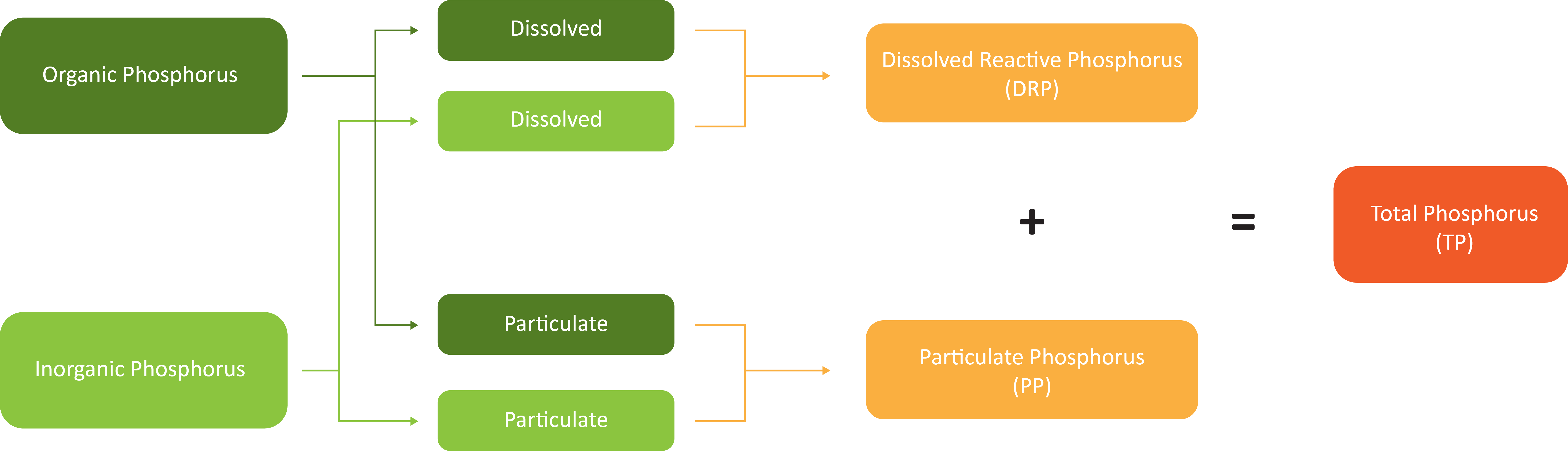
When a water sample is analysed for phosphorus, different techniques are applied to isolate the various forms.
Phosphorus is typically analysed and reported as:
- Total Phosphorus (TP)
- Dissolved Reactive Phosphorus (DRP)
- Particulate Phosphorus (PP) (PP = TP – DRP)
For more information on water quality contaminants see Nitrogen, Sediment, and Microbes.