Nitrogen
Nitrogen (chemical symbol N) has three main forms in the environment: molecular, organic, and inorganic.
Molecular nitrogen is a gas (N₂) and makes up about 78% of the Earth\‘s atmosphere.
Organic nitrogen refers to the diverse range of nitrogen-containing organic molecules, such as simple amino acids, proteins, and nucleic acids, to large and complex molecules, such as humic substances (or plant material) in soil and water.
Inorganic nitrogen occurs in three forms:
Nitrate (NO₃⁻) is the preferred form of nitrogen nutrition for most species of plants. Nitrate is highly soluble and is easily transported through the soil if not used by plants and microorganisms. Sources of nitrate include inorganic fertiliser, animal wastes including farm dairy effluent, septic tanks and sewage systems. Nitrate also occurs as a result of nitrification of the ammonium in animal waste by bacteria in the soil. It is toxic at high concentrations. The majority of nitrate is released through microbial mineralisation processes irrespective of the form of input.
Nitrite (NO₂⁻) is formed during the process of nitrification but its concentration is often low compared to other forms of inorganic nitrogen.
Ammoniacal nitrogen (NH₄⁺/NH3(g)) is represented by ammonia (NH₃) and ammonium (NH₄⁺). Which form dominates in water is dependent on pH, with ammonia concentrations increasing as pH increases. In most natural waters with pH values less than 7.5, ammonium is the dominant form. Ammonium is less mobile than nitrate as it is strongly attracted to negatively charged clay minerals. Where it occurs, ammonia is highly toxic to fish and other aquatic organisms.
What forms of nitrogen are ecologically important?
The organic and inorganic forms of nitrogen are ecologically important. It is these compounds that are added as nutrients (i.e., fertilisers) to enhance plant growth. However, as they are highly soluble, they can easily become environmental contaminants in the wrong location. In surface water, this can cause nuisance aquatic weeds and algae to flourish.
Nitrite and ammoniacal nitrogen both become toxic at high concentrations.
How is nitrogen cycled on my farm?
Nitrogen in the environment is cycled from one form to another depending on the environmental and biological conditions.
In a farm system, the primary inputs of nitrogen to the soil are effluent from livestock, plant materials (organic matter), and synthetic fertiliser. There will also be minor contributions from the atmosphere. Fertiliser and urine inputs are generally in the form of urea ((NH₂)₂CO), which is quite rapidly converted to ammonium and ammonia (collectively they are known as ammoniacal nitrogen). Regardless of the source of nitrogen, the processes in the nitrogen cycle always apply.
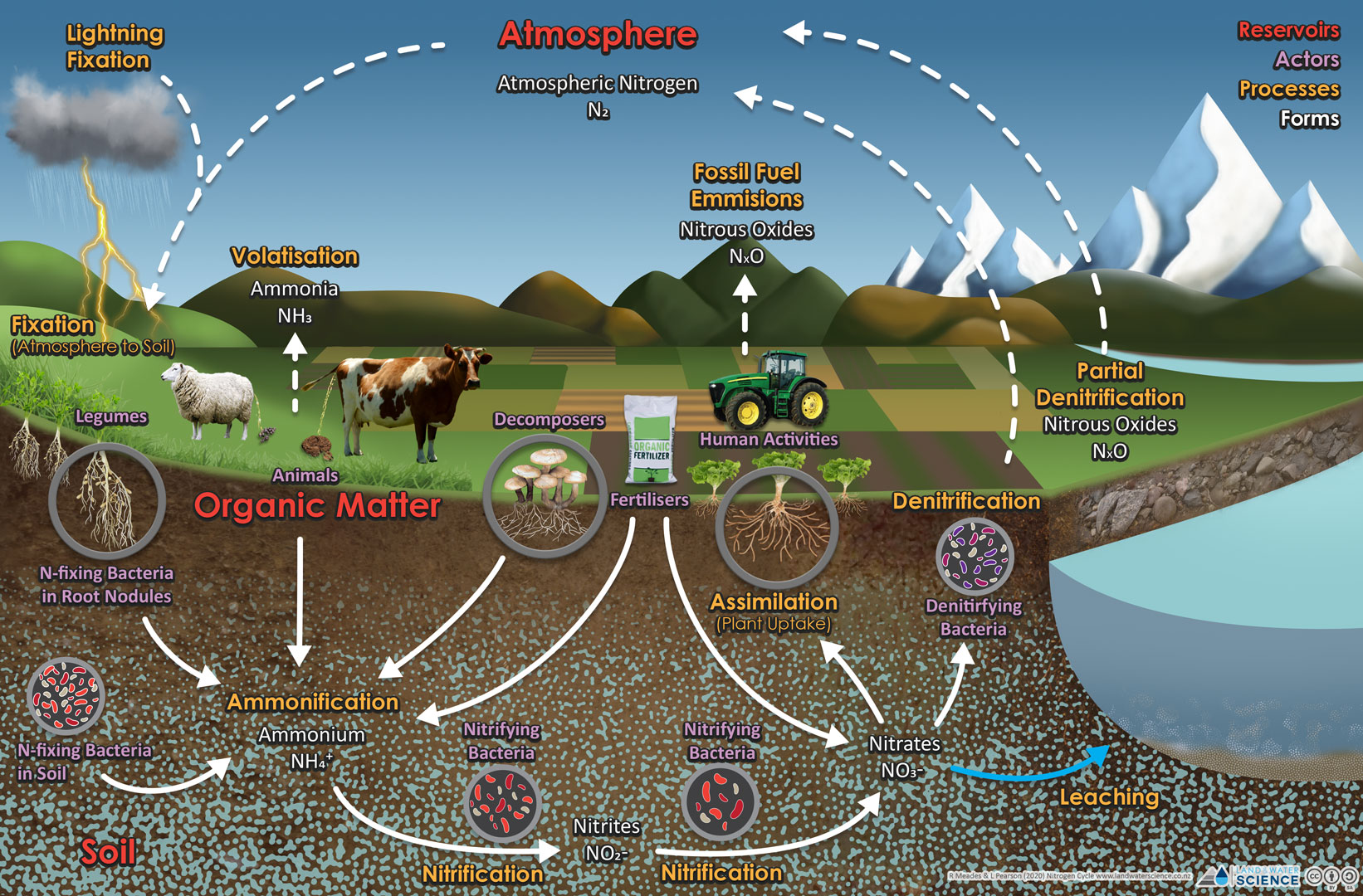
The two main forms of inorganic nitrogen in the soil are ammonium (NH₄⁺) and nitrate (NO₃⁻). Ammonium and ammonia co-exist, with the proportion of each dependent on pH, soil temperature, and moisture. Ammonia may be lost to the atmosphere in response to volatilisation. In addition to fertiliser and urine, ammoniacal nitrogen is released during the breakdown of organic matter (which can be dung or dead plant material). In response to breakdown, mineralised nitrogen may be sequestered or released by the microbial biomass into solution. The conversion from ammonium to nitrate (nitrification) is a biologically mediated process involving microorganisms that is also influenced by soil pH, moisture, and temperature.
Ammonium and nitrate are both available for plant uptake, but there are important differences in their characteristics. Nitrate is highly mobile in soil due to its negligible adsorption characteristics, but ammonium is generally much less mobile because it tends to adsorb to the soil particles, particularly in soils with a high clay content. Nitrate is subject to denitrification, which is the gaseous loss of nitrogen as both nitrogen gas (N₂) and nitrous oxide (N₂O, a harmful greenhouse gas). If nitrate is not denitrified to gaseous forms, it can be lost to water through leaching. Ammonium is also lost to the atmosphere by volatilisation through the emission of ammonia gas (NH₃) (This is the smell we associate with urine). Both denitrification and volatilisation processes respond to short-term daily climate and soil factors and can be highly episodic.
In most mineral or non-wetland soil types, the dominant form of nitrogen below the root zone is nitrate nitrogen, with a potentially important contribution from small dissolved organic nitrogen forms. Other forms of nitrogen, particulate organic N, larger dissolved organic forms, and ammonium seldom percolate to these depths due to physical exclusion (filtering), they are held by the soil or plant roots, and other biogeochemical processes. These forms of nitrogen tend to accumulate at or near the soil surface and can be more easily transported with runoff.
For artificially drained soils (mole-pipe drainage type), the potential range of nitrogen forms transported during drainage will vary according to the soil\‘s carbon content, soil water residence time, and the effectiveness of the drainage system.
How do I know what form of nitrogen is being lost from the land?
In areas dominated by mineral soils, the main form of nitrogen is typically nitrate. These areas have the highest inherent nitrate risk for loss and are often associated with well drained soils and shallow alluvial aquifers that occur across a catchment. In these areas the land has little to no ability to remove nitrate naturally. Organic and ammoniacal nitrogen are often a minor component of total nitrogen in these soils. However, they can be significant during periods of heavy rainfall. Runoff events are known to supply significant amounts of organic and ammoniacal nitrogen to a stream network.
Where there are organic soils (peat), the dominant form of nitrogen is usually organic. When this land is used for pastoral grazing, ammoniacal nitrogen is often the form that is most mobile and easily lost. This is because these soils are naturally wetter, and runoff can occur more frequently. The landscape in this setting has a naturally high ability to remove nitrate nitrogen by denitrification.
How is nitrogen measured in water?
When a water sample is analysed for nitrogen, different techniques are applied to isolate the various forms.
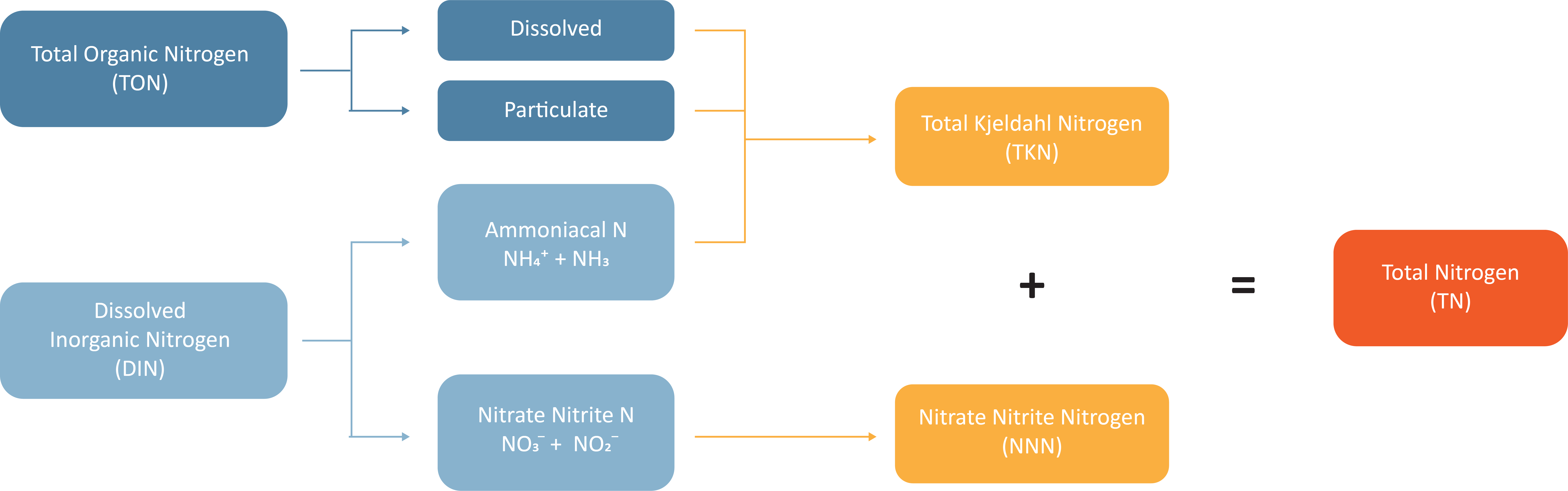
Nitrogen is typically analysed and reported as follows:
Nitrate-Nitrite Nitrogen (NNN) = Nitrate + Nitrite
Total Kjeldahl Nitrogen (TKN) = Total Organic Nitrogen + Total Ammoniacal (NH₃ + NH₄⁺)
Dissolved Inorganic Nitrogen (DIN) = Nitrate-Nitrite Nitrogen + Total Ammoniacal (NH₃ + NH₄⁺)
Total Nitrogen (TN) = TKN + NNN
For more information on water quality contaminants see Phosphorus, Sediment, and Microbes.